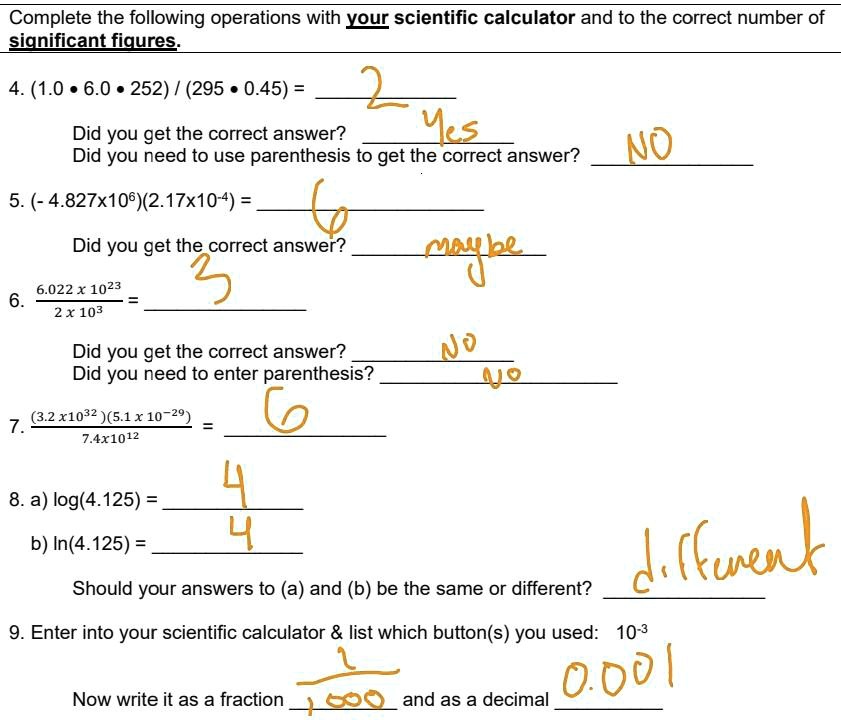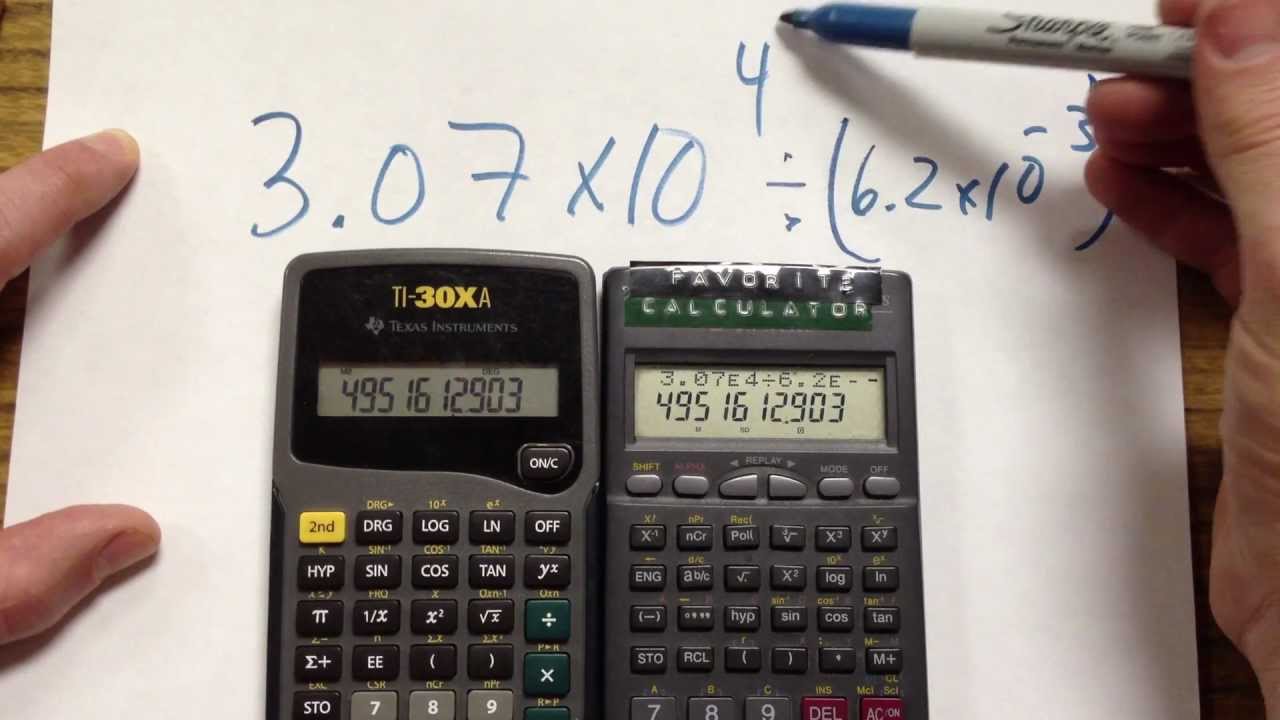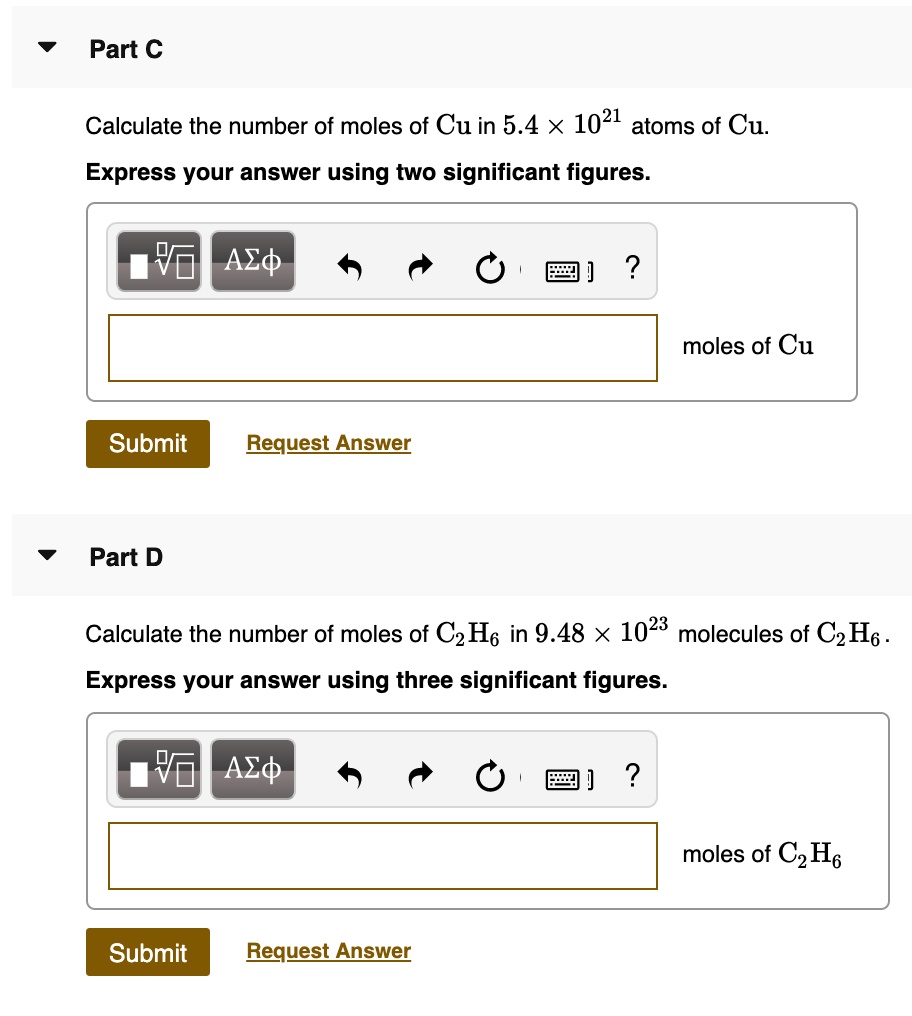
SOLVED: Part C Calculate the number of moles of Cu in 5.4 X 1021 atoms of Cu: Express your answer using two significant figures Azd moles of Cu Submit Request Answer Part

12 g C - 12 contains 6.022 × 10^23 atoms of carbon.(a) 6.022 × 10^23 is known as .............(b) Calculate the number of carbon atoms present in 48 g C - 12.(c)

How to Calculate Total Charge in Coulombs of an Arrangement of Protons and Electrons | Physics | Study.com

Silver metal crystallizes with a face - centred cubic lattice. The length of the unit cell is found to be 3.0 × 10^-8 cm . Calculate atomic radius and density of silver. (